
The United States has made enormous progress in reducing water pollution since the Clean Water Act was passed nearly 50 years ago. Rivers no longer catch fire when oil slicks on their surfaces ignite. And many harbors that once were fouled with sewage now draw swimmers and boaters.
But new, more complex challenges are emerging. In a study published earlier this year, we found that a cocktail of chemicals from many human activities is making U.S. rivers saltier and more alkaline across the nation. Surprisingly, road salt in winter is not the only source: construction, agriculture, and many other activities also play roles across regions.
These changes pose serious threats to drinking water supplies, urban infrastructure and natural ecosystems. Salt pollution is not currently regulated at the federal level, and state and local controls are inconsistent.
Our research shows that when salts from different sources mix, they can have broader impacts than they would individually. It also shows the importance of supporting water quality monitoring nationwide, so that we can detect and address other pollution problems that have yet to be recognized.
Altered waters
Our group has been studying freshwater salinization for over 15 years. In 2005 we published a paper that demonstrated that levels of sodium chloride (common table salt) were rapidly increasing in fresh waters across the northeastern United States.
Until that time, scientists thought that salinization was a serious problem mainly in arid regions where water evaporates rapidly, leaving salts behind. But we found that it was affecting major drinking water supplies, exceeding toxic levels for some aquatic organisms and persisting in the environment year-round, even in humid regions.
The main cause we found was the spread of paved surfaces, such as roads and parking lots. Communities in cold regions use de-icing salts to clear snow from roads during winter, and the more roads they build, the more treatment is needed. We found that a 1 percent increase in paved surfaces could boost salt concentrations in nearby water bodies to levels more than 10 times higher than pristine forested conditions.
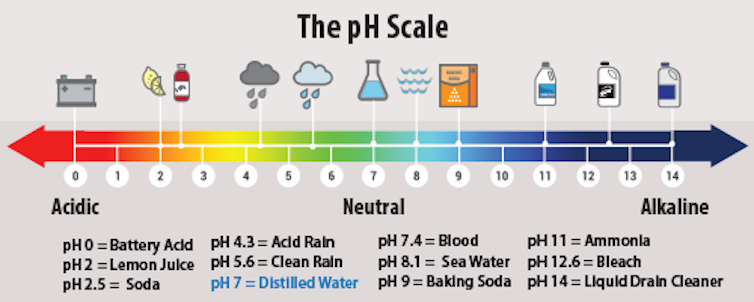
In 2013, we published another study showing that rivers were becoming more alkaline across regions of the eastern United States. At that time acid rain – i.e., too much acid in rainwater, caused by air pollution – had been a well-known environmental issue for several decades. However, alkalinization was not recognized in the same way, and its effects are still poorly understood now.
Alkalinization is the opposite of acidification: It occurs when water’s pH value increases instead of falling. As water becomes more alkaline, certain chemicals dissolved in it can become toxic. For example, ammonium is a nutrient in freshwater ecosystems, but is converted to toxic ammonia gas in significant concentrations in waters with a high pH. Alkaline conditions also enhance release of phosphorus from sediments, which can trigger nuisance blooms of algae and bacteria.
We found that a process we called “human-accelerated weathering” was breaking down rock and releasing minerals into rivers that were making them more alkaline. The process of weathering rocks and minerals that become exported to rivers is typically slow, but we showed that land development and decades of exposure to acid rain were speeding it. We also suggested that widespread use of geologic materials in fertilizers and concrete was a factor.
Identifying freshwater salinization syndrome
Our study on human-accelerated weathering showed that along with sodium chloride, other dissolved salts were increasing in fresh water across large regions of the eastern United States. This made us wonder whether there could be a link to our previous work on salinization in these regions.
We started to recognize that in theory, salt pollution and human-accelerated weathering could be sending increasing quantities of salts that were alkaline into rivers throughout the nation, and that this could increase their pH levels. We knew that ocean water, which is naturally salty, has a higher pH than fresh water because it has accumulated high levels of alkaline salts. After much analysis, we proposed that similar interconnected processes could influence salinity and pH in fresh water.
Many sources release alkaline salts into the environment, including weathering of impervious surfaces, fertilizer and lime use in agriculture, mine drainage, irrigation runoff and winter use of road salt. Initially, parts of these alkaline salts bind to soil. But when they come into contact with sodium – for example, excess road salt – chemical reactions occur that release the alkaline salts, which then wash into freshwater ecosystems.
We called this process freshwater salinization syndrome because it was producing multiple effects on salts, alkalinity and pH, which are fundamental chemical properties of water.
Different causes by region
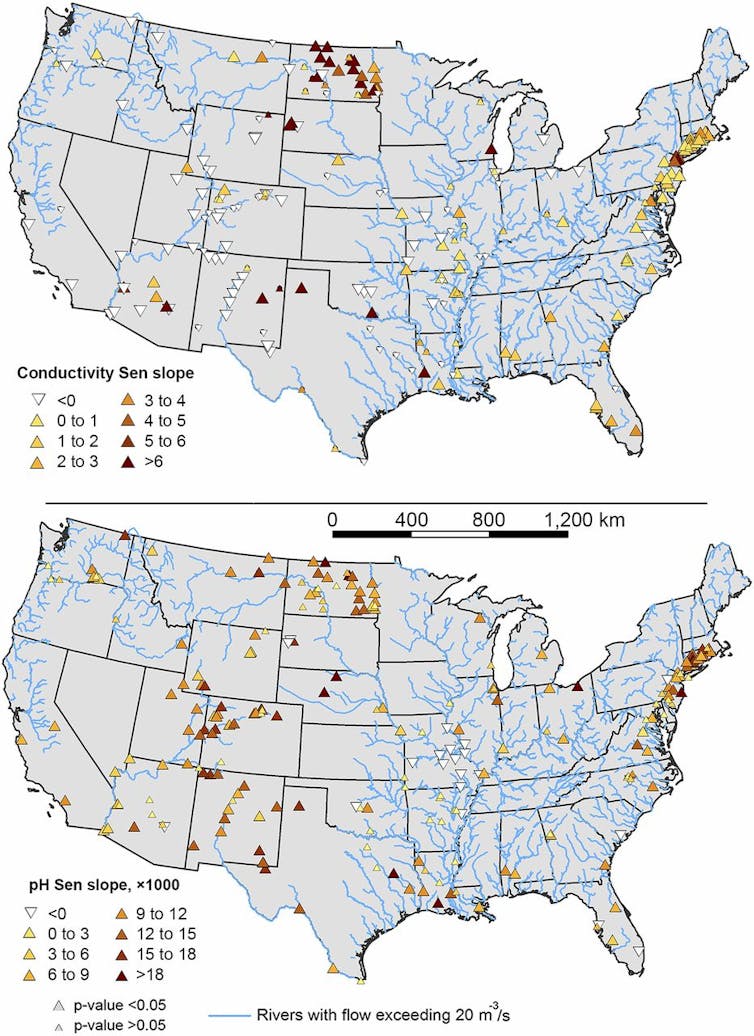
Figuring out this process was a team project that required knowledge of limnology (the study of inland waters), geochemistry and geography. The causes vary from one location to another, but the outcomes can be similar.
For example, rivers are becoming more saline and alkaline in parts of North Carolina, Florida, Virginia and other states that use little or no road salt. This is likely due to human-accelerated weathering in locations underlain by limestone (which dissolves when it comes in contact with acid rainwater) and in urbanized areas with lots of concrete infrastructure, as well as urban salt pollution from sewage, water softeners or fertilizers.
Our research was supported by the U.S. National Science Foundation and drew on enormous quantities of monitoring data from ecosystems across the United States collected mainly by the U.S. Geological Survey. We analyzed long-term trends in the chemistry of rivers over five decades and compared these trends across different major river systems and regions.
We also analyzed trends in major estuaries, such as the Hudson River and the Chesapeake Bay, to investigate whether increasingly alkaline inputs from rivers could potentially influence the chemistry of coastal waters. Our results show that changes in salts can alter concentrations of pollutants such as excess phosphorus and nutrients that are bound up in sediments at these sites.
Managing salt pollution
Freshwater salinization syndrome is affecting drinking water supplies in many parts of the United States. In some cases it is altering the taste of water or threatening the health of people with hypertension.
There is growing concern that salts in fresh water can corrode water pipes and release toxic metals such as lead into drinking water. They also can trigger reactions that mobilize other contaminants and pollutants from soils into rivers.
As other scientists have shown, mixtures of salts can be more toxic to aquatic life than just one salt alone. The Environmental Protection Agency does not currently regulate salts as primary contaminants in drinking water, and state and local regulation of salt releases over wide areas from activities such as road treatment are sparse and inconsistent.
We believe there is a serious need for federal regulations and regional plans to reduce salt pollution in fresh water. One strategy would be to reduce use of road salts by calibrating application and adjusting application rates based on temperature. In addition, not all salts are created equal: It may be more efficient to use certain salts as deicers at lower temperatures. Finally, organic de-icing solutions use less salt than conventional versions.
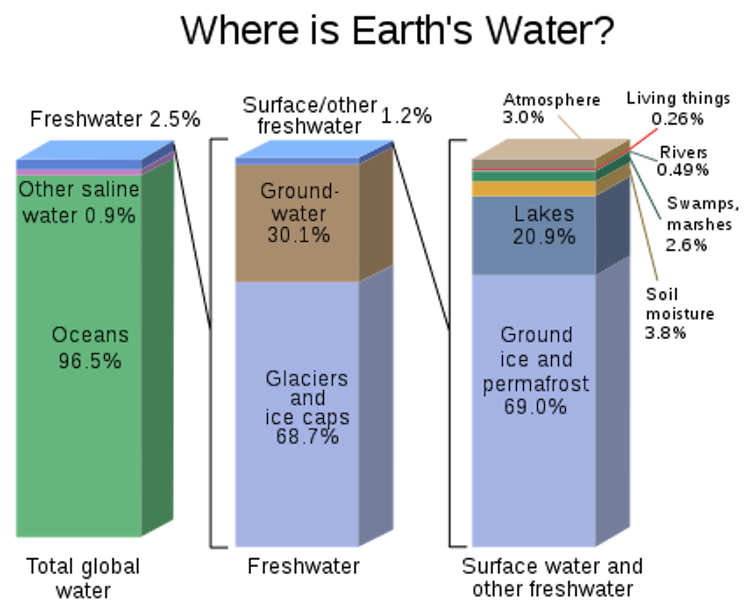
![]() New forms of water pollution are constantly emerging, and it is important to identify how different human activities accelerate geological processes in nature. Fresh water accounts for only about 3 percent of the Earth’s total water supply (the rest is in the oceans), and there will always be a need for better understanding and management of this precious resource.
New forms of water pollution are constantly emerging, and it is important to identify how different human activities accelerate geological processes in nature. Fresh water accounts for only about 3 percent of the Earth’s total water supply (the rest is in the oceans), and there will always be a need for better understanding and management of this precious resource.
Sujay Kaushal, Associate Professor of Geology, University of Maryland; Gene E. Likens, Distinguished Research Professor of Ecology and Evolutionary Biology, University of Connecticut; Michael Pace, Professor of Environmental Sciences, University of Virginia, and Ryan Utz, Assistant Professor of Water Resources, Chatham University
This article was originally published on The Conversation.
